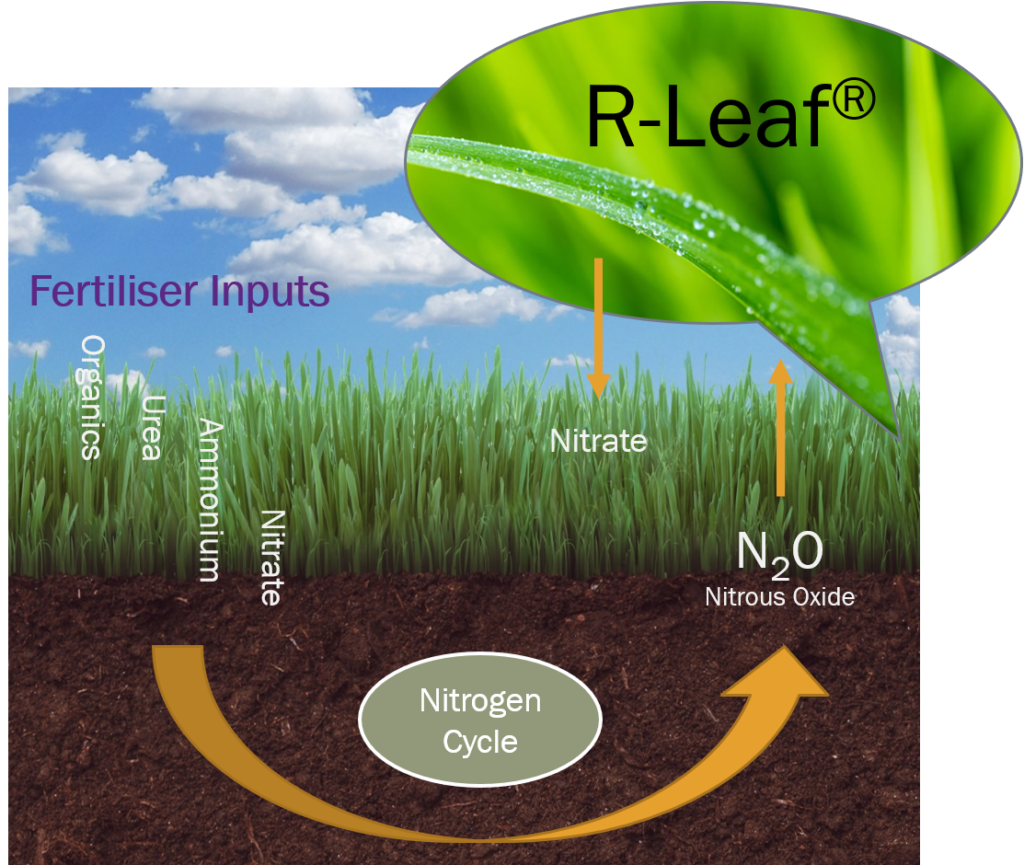
What is R-Leaf®
A disruptive technology developed by Crop Intellect that captures atmospheric NOx pollutants (N2O, NO, NO2) and converts them to plant feed. The technology is prepared into a suspension concentrate solution and it is sprayed on living plant surfaces. The NOx pollution is broken down to nitrate which is absorbed by the plants as feed. This results in reduced air pollution and increased crop yield.
How to use R-Leaf® in farming?
R-Leaf® is sprayed on any crop by typical spraying equipment. It is a stable suspension concentrate and can be tank mixed with most agrochemical inputs. Contains 500g of the specially processed photocatalytic material. It is classed as EC fertiliser containing Zn, Mo and Mn which are very often found in deficiency, particularly in cereals and are essential elements for yield security.
The recommended dosage of R-Leaf® is 1lt/ha. The best application timing for cereals is at T1 and T2 when the soil applied nitrogen is starting to produce NOx and when the foliage is adequate to hold R-Leaf®. The T2 application is essential as the sprayed leaves are shaded by the new ones and therefore become less efficient.
R-Leaf® captures soil derived NOx and from the air and converts it to nitrate. This process occurs daily on the surface of sprayed plants resulting in a supply of nitrogen to the crop daily. This can allow a reduction in the application of bulk nitrogen used.
R-Leaf® provides further benefits to plants which are not explained here but contribute towards a higher yielding crop.
Can R-Leaf® be used to obtain carbon credits?
Crop Intellect Ltd is in the process of registering the application of R-Leaf® as a project for obtaining carbon credits. When this is completed the end user will be able to claim carbon credits for every hectare sprayed. This will provide a monetary value and contribute towards net zero emission targets.
Businesses who would like to claim the carbon credits produced, could subsidise the use of R-Leaf® in farming which will make farming more profitable through increased yield and nitrogen use efficiency.
The environmental and health benefit of R-Leaf® will be appreciated and enjoyed by everyone!
Who funded the development of R-Leaf®?
Crop Intellect was successful in obtaining funding from InnovateUK, Climate-KIC, EIC Accelerator (EU Funding) and others to perform the research for the development of R-Leaf®. Over £0.5m have been invested to date for the research and development, including trials of R-Leaf® to demonstrate its efficacy. A further £1.5m have been committed by the EIC Accelerator Programme and Crop Intellect to commercialise the technology.
R-Leaf removes the equivalent of 5.4 tCO2 per year used at 2 litres per hectare on cereals as validated by the Climate Impact Forecast (CIF) tool supported by the European Innovation Council. The impact is valid, positive and significant.

Technical Information on R-Leaf®
Please download PDF file Technical Info on R-Leaf HERE
How does R-Leaf® work?
R-Leaf® is made up of photocatalysts that have been specially processed to enable NOx catalysis under normal day light instead of the need of high intensity light i.e. UV. The R-Leaf® particles remain on the surface of sprayed plants and work tirelessly on a daily basis to break down NOx into nitrate which is the most available form of nitrogen for plants. The nitrate is solubilised with dew and rain and taken up by the plant leaves resulting in increased biomass.
When the photocatalysts in R-Leaf® are charged with day light, electrons and holes are produced. The negatively charged electrons capture an oxygen from the air and produce superoxide anions. Holes (h+) capture water molecules from the air and produce hydroxyl radicals (.OH). In contact with NOx they react and break NOx down to nitrate, water and carbon dioxide. These components are essential for crop growth.
What is photocatalysis and how it works
Photocatalysis is the activity taking place when an intense light source interacts with the surface of semiconductor materials, so called photocatalysts. The photocatalysts used in R-Leaf® have been processed to harvest energy from day light which is free, clean and in abundance.
Photocatalysts have been studied for over 100 years and are well understood, proven, and used currently in many industries as filters, decontaminants, and chemical reactors. In R-Leaf®, simply explained, day light charges the photocatalytic surface and when NOx come in contact they break down to inert material and nitrate. The photocatalyst itself undergo no changes and therefore continues to work for a prolonged period.
The photocatalysts utilised are chosen for their suitability and selectivity for NOx, availability for scaling up, low cost, chemical stability, and safety.
What are NOx and where do they come from?
NOx pollution occurs when nitrogen oxides are released as a gas into the atmosphere during the high-temperature combustion of fossil fuels. These nitrogen oxides consist mainly of two molecules, nitric oxide (NO) and nitrogen dioxide (NO2); there are other nitrogen-based molecules considered to be NOx, but they occur in much lower concentrations. A closely related molecule, nitrous oxide (N2O), is a significant greenhouse gas that plays a role in global climate change.
The continuous increase of its concentration, both due to natural and anthropogenic sources (use of synthetic fertilizers, adipic acid production, nitric acid production, fossil fuels and biomass burning) and long atmospheric residence time (150 years), entails the need of developing efficient method for its abatement. N2O decomposition into nitrogen and oxygen offers simple solution for its conversion to natural components of air. Limiting the formation of N2O is the best solution to reduce N2O emissions from the agricultural sector and uncontrolled biomass burning considering the diffuse character of these emissions, while employment of after treatment technologies is important for control of N2O emissions from combustion and industrial sources.
N2O is also a by-product from nitric acid production, which is critical to produce nitrate fertilizers and for several important industrial chemicals. Since N2O has a global warming potential of 265 times more than CO2, the reduction of N2O emissions is of utmost importance for this industry. By pursuing innovative technological solutions such as R-Leaf, reduction of over 75% of N2O emission could be achieved with a wide-scale implementation.
How is NOx measured?
The concentration of NO2 is measured in micrograms in each cubic metre of air (μg m-3). To protect our health the UK Governments set two air quality objectives, 1. The hourly objective, which is the concentration of NO2 in the air averaged over an hour. This ensures that the exposure to high concentrations is monitored; 2. The annual objective which is the concentration of NO2 in the air averaged over a year. This is to protect us from long term exposure.
The European Union (EU) has set the exposure limits of NO2 to 200 μg m-3 hourly with no more exceedances over a year and the annual limits to 40 μg m-3. NOx can be measured continuously using an instrument called chemiluminesence analyser or cumulatively using NOx diffusion tubes.
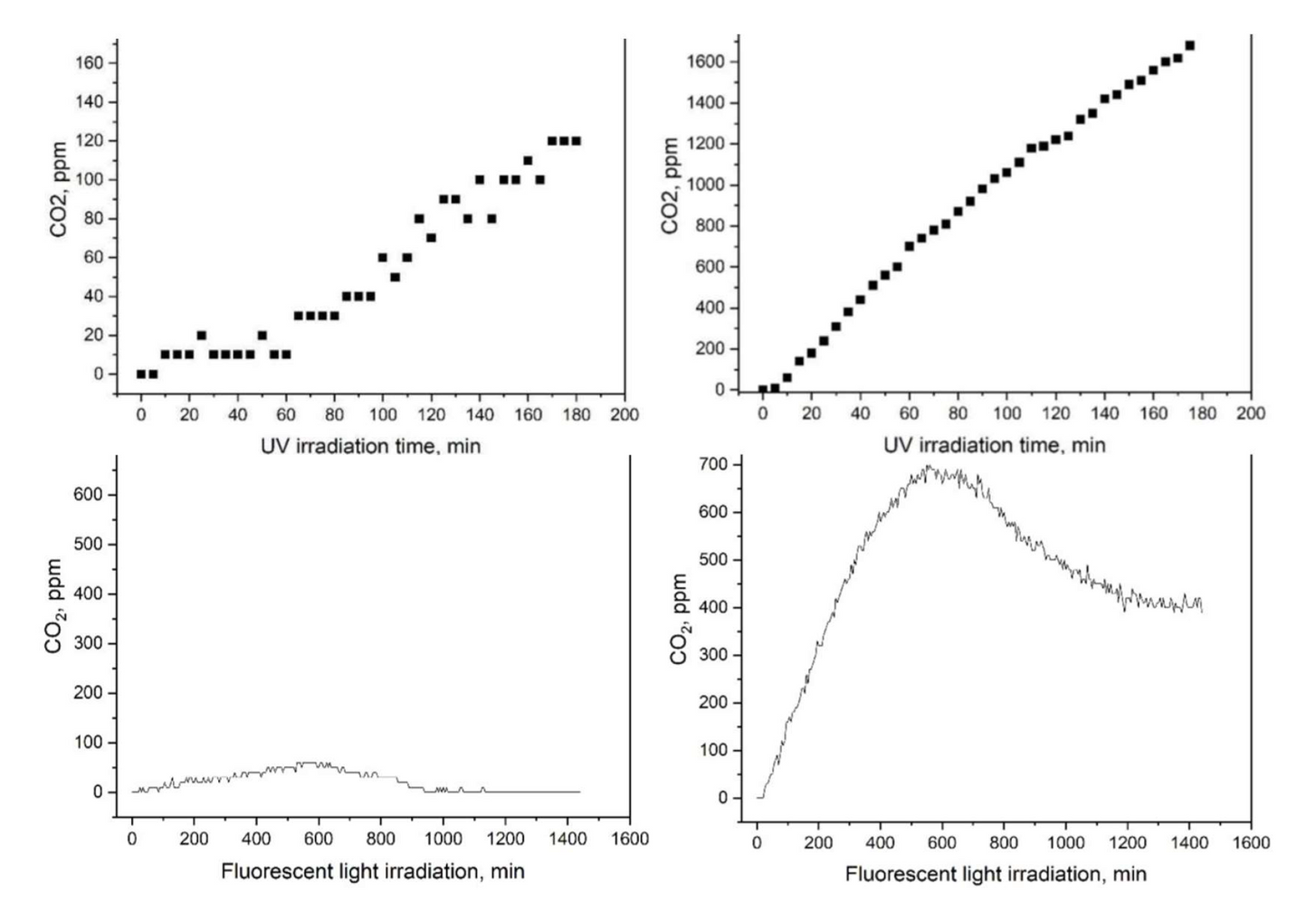
Evidence of R-Leaf® efficacy?
The photocatalytic activity of R-Leaf® under UV and normal light was tested by independent experts in photocatalysis at Manchester Metropolitan University. The approved method measuring photocatalysis was by monitoring the CO2 in a specific reaction and results are shown below. The material was 10 times more effective in photocatalysis, both under UV light and normal light compared to the unprocessed material. Note that CO2 is not produced by R-Leaf®.
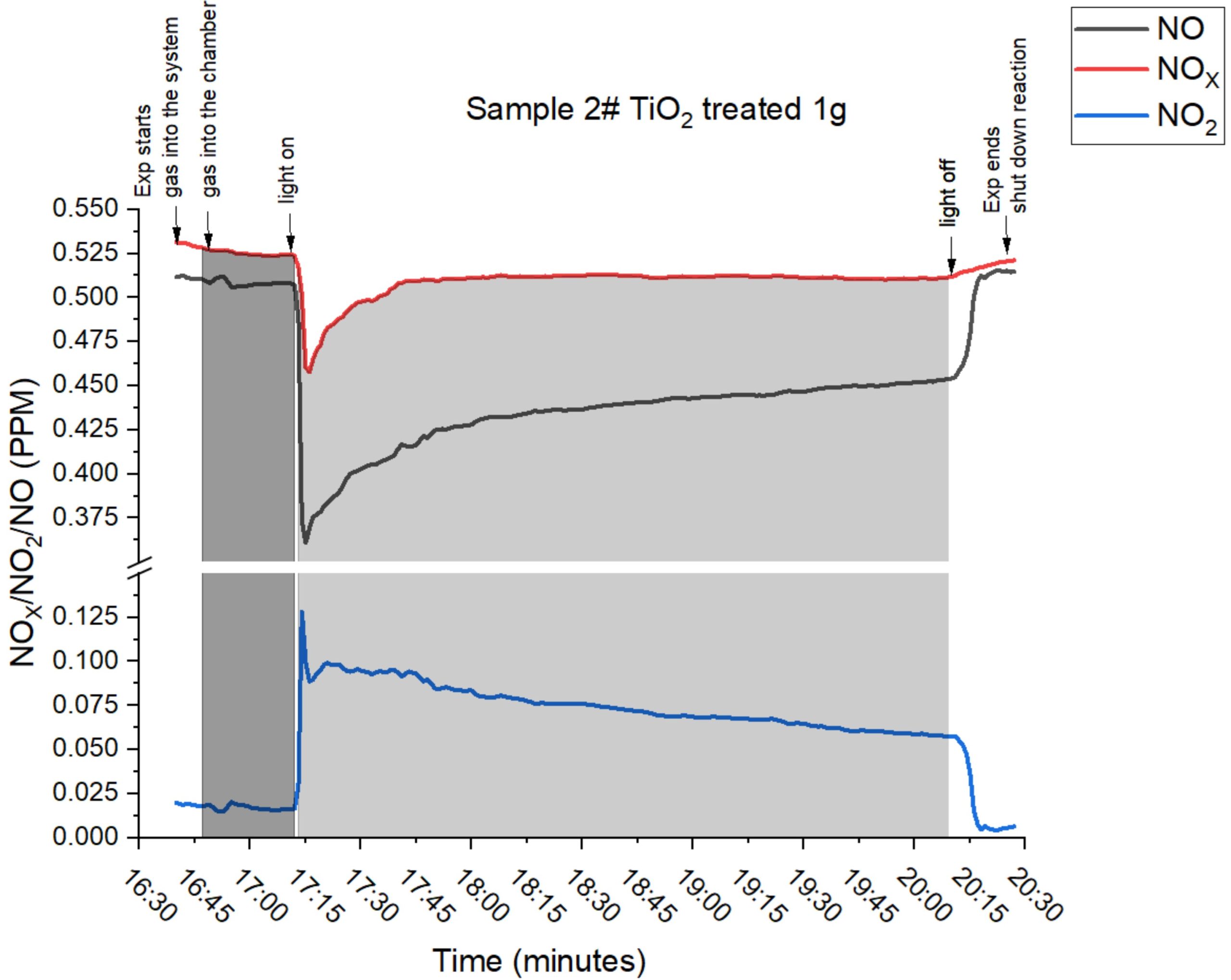
Data generated in collaboration with the University of Lincoln confirmed the conversion of N2O, NO and NO2 to nitrate for plant use. The amount of nitrate produced is directly related to the ppm NOx concentration.